Proactive Risk Management
Stop Managing Chaos. Start Engineering Certainty.
In complex food and beverage product development, identifying risk is only half the battle. The true differentiator is having a system that quantifies that risk, enforces a clear response, and provides executive visibility. Fulvisol integrates powerful risk management tools directly into your New Product Introduction (NPI) process, moving risk analysis out of siloed spreadsheets and into your Digital Thread. This allows you to convert potential failure—from raw material traceability issues, FSA compliance, or manufacturing contamination risks—into engineered confidence.
Quantification & Prioritisation: The RPN Core
Our system replaces subjective guesswork with verifiable, quantitative scoring, ensuring your teams focus their limited resources on the highest-impact threats to consumer safety and market launch.
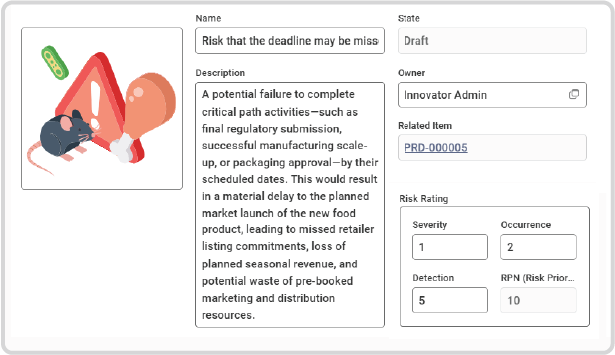
Guided Risk Capture:
Easily document any potential threat—from allergen cross-contamination during scale-up to supplier failure to meet ethical sourcing standards—with a dedicated, structured workflow.
Objective RPN Calculation:
Define and score each risk using three critical dimensions:
– Severity: The magnitude of impact if the risk occurs (e.g., product recall severity).
– Occurrence: The probability of the risk happening (e.g., historical supplier failure rate).
– Detection: The likelihood of identifying the risk before it leaves the factory (e.g., effectiveness of quality gate controls).
Clear Prioritisation:
The system automatically calculates the Risk Priority Number (RPN), providing an objective metric to help identify the need for resource allocation and management focus.
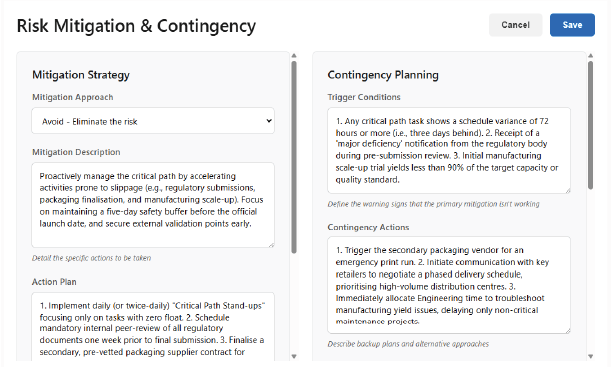
Strategy & Resilience: Mitigation and Contingency
A comprehensive risk plan requires both preventative measures and recovery protocols. Fulvisol facilitates the definition of robust, measurable response strategies for every threat.
Mitigation Strategy:
Detail the specific actions required to reduce the Occurrence or Severity of the risk (e.g., qualifying a secondary ingredient supplier, enforcing HACCP checks, or introducing a new ingredient inspection gate).
Contingency Actions:
Clearly outline the recovery steps, resources, and leadership required to minimise damage if the risk materialises (e.g., initiating a containment procedure or negotiating alternative distribution channels), ensuring a fast, controlled response rather than panic.
Compliance at the Source:
By integrating these rules into the PLM environment, you ensure that product packaging, user manuals, and technical specifications are automatically aligned with the latest brand standards before market launch.
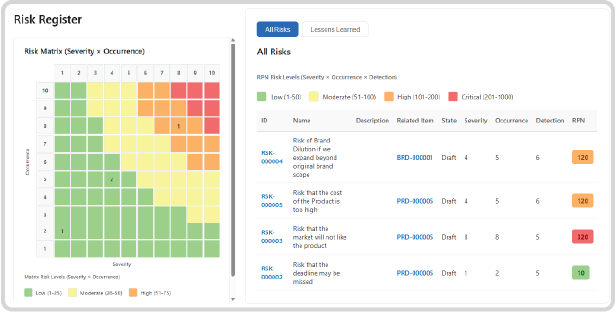
Comprehensive Visibility: The Risk Register
Executives and Project Managers need immediate insight, not dense reports. Our integrated Risk Register transforms complex data into immediate, actionable views for the NPI portfolio.
Risk Matrix Visualisation:
Instantly see the entire risk portfolio plotted on a dynamic matrix (Severity vs. Occurrence), allowing leadership to visually track high-exposure risks (the “Red Zone”), such as major compliance failures or high-impact ingredient price hikes.
Real-Time Risk Table:
Access a detailed, filterable table that captures status, ownership, RPN history, and the date of the last review for every identified risk.
Lessons Learned:
Capture the outcome of every risk event (or successful mitigation) and link it back to the originating product stage. This creates a valuable knowledge base for future New Product Introduction (NPI) cycles, driving continuous process improvement across the entire organisation.
Interested in seeing what Fulvisol Food + Beverage could do for your business?
Streamline the transition from R&D and pilot production to full-scale manufacturing
By submitting this form I agree to my details being used in sole connection with the intended enquiry. Please check our privacy policy to see how we protect and manage your submitted data.