Packaging Compliance, Design, and Speed to Shelf
From Material Composition to Final Artwork: Automated Precision.
Packaging is the intersection of consumer promise, legal compliance, and product safety. Manually compiling pack copy, verifying material safety, and managing design revisions are major sources of product launch delay and regulatory risk. Fulvisol’s Packaging Management creates a seamless, compliant workflow, ensuring every element – from the material composition to the printed barcode – is accurate, approved, and auditable.
Material Compliance and Environmental Footprint
Control your primary and secondary packaging materials with the same rigour as your food ingredients.
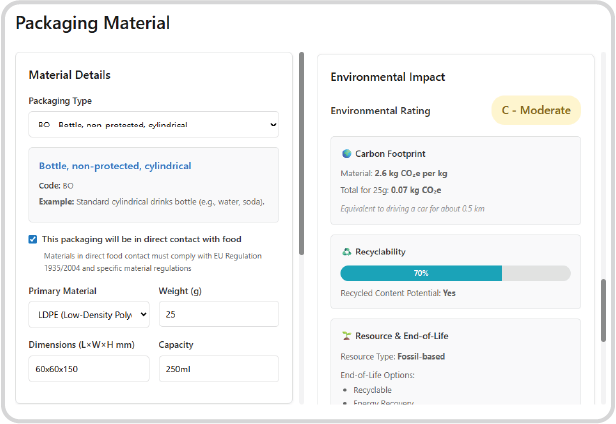
Material Composition Definition:
Define the precise materials used, including complex material composition. This foundational data is critical for both regulatory checks and sustainability reporting.
Automated Food Contact Safety:
Specify if the material will be in direct contact with food. Fulvisol automatically checks compliance against critical food contact regulations, including the General Framework for Food Contact Materials, Plastic Materials and Articles Regulations, and Good Manufacturing Practice (GMP) guidelines.
Environmental Impact Assessment:
Leverage the material composition data to automatically assess the packaging’s recyclability and environmental impact. This drives sustainable material selection and provides auditable data for required Extended Producer Responsibility (EPR) reporting.
Pack Copy Automation and Mandatory Checkpoints
Eliminate the risk of publishing incomplete or non-compliant product information by leveraging verified data from the Digital Thread.
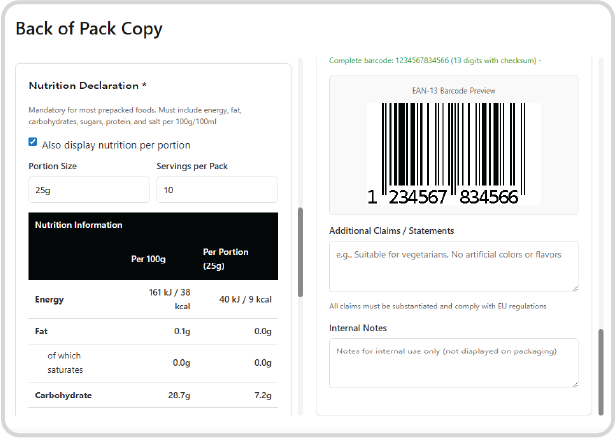
Data-Driven Copy Generation:
Automatically pull all necessary product information—including declarations, ingredient lists, nutritional panels, and manufacturer addresses—directly from the approved Recipe or related Trade Item. This builds a reliable starting point for your BOP (Back of Pack) and FOP (Front of Pack) copy.
Mandatory Information Validation:
The system performs a regulatory check to ensure all mandatory information is present and accurately defined in the FOP and BOP copy for the target market. You cannot proceed until all required fields are specified and approved, preventing critical compliance failures at launch.
Barcode Generation:
Barcodes are automatically generated and validated using the Global Trade Item Number (GTIN) from the related Trade Item, ensuring scannability and data integrity at the point of sale.
The Pack Copy Master: Visualising Compliance on the Die-Line
Bridge the gap between compliance text and design layout by providing designers with a smart, pre-validated starting point.
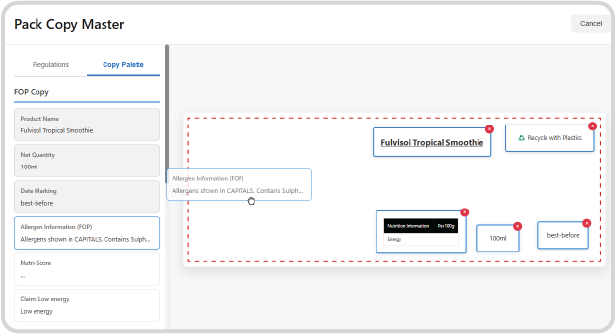
Die-Line Integration:
Upload the designer-provided die-line template (the net shape of the packaging).
Copy Palette Placement:
Using the Pack Copy Master tool, teams can drag and drop representative text blocks and legal information from the compliance-validated Copy Palette onto the die-line.
Indicative Layout:
This provides an indicative starting point for your design agency, demonstrating the required size, placement, and spatial allocation for regulatory text and mandatory symbols, drastically cutting down on design revisions and ensuring legal copy fits.
Artwork Markup and Auditable History
Streamline the final artwork review process with integrated annotation tools and maintain a bulletproof record of all changes.
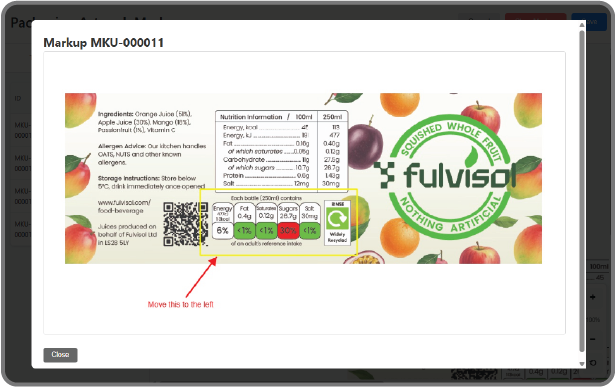
Digital Markup Tools:
Review and collaborate directly on the final packaging artwork file. Use built-in annotation tools—including arrows, drawings, boxes, and text markups—to communicate necessary changes and corrections instantly.
Artwork Markup History:
Every annotation, comment, and revision is automatically captured and added to an Artwork Markup History. This creates a clear, traceable record of who made the changes, when they were made, and why (the rationale behind the markups), establishing a comprehensive audit trail for quality assurance.
Interested in seeing what Fulvisol Food + Beverage could do for your business?
Streamline the transition from R&D and pilot production to full-scale manufacturing
By submitting this form I agree to my details being used in sole connection with the intended enquiry. Please check our privacy policy to see how we protect and manage your submitted data.